Nicotine constitutes approximately 0.6–3.0
% of the dry weight of tobacco.
[11] Usually consistent concentrations of nicotine varying from 2–7
µg/
kg (20–70 millionths of a percent wet weight) are found in the edible family
Solanaceae, such as
potatoes,
tomatoes, and
eggplant.
[12] Some research indicates that the contribution of nicotine obtained from food is substantial in comparison to inhalation of
second-hand smoke.
[12] Others consider nicotine obtained from food to be trivial unless exceedingly high amounts of certain vegetables are eaten.
[12] It functions as an
antiherbivore chemical; consequently, nicotine was widely used as an
insecticide in the past,
[13][14]and
neonicotinoids, such as
imidacloprid, are widely used.
Nicotine is highly
addictive.
[15][16][17] It is one of the most commonly abused drugs.
[18] An average
cigarette yields about 2 mg of absorbed nicotine; high amounts can be harmful.
[19] Nicotine induces both behavioral stimulation and anxiety in animals.
[5] Nicotine
addiction involves drug-reinforced behavior, compulsive use, and relapse following abstinence.
[20] Nicotine
dependence involves tolerance, sensitization,
[21] physical dependence, and
psychological dependence.
[22] Nicotine dependency causes distress.
[23][24] Nicotine withdrawal symptoms include depressed mood, stress, anxiety, irritability, difficulty concentrating, and sleep disturbances.
[1] Mild nicotine withdrawal symptoms are measurable in unrestricted smokers, who experience normal moods only as their blood nicotine levels peak, with each cigarette.
[25] On quitting, withdrawal symptoms worsen sharply, then gradually improve to a normal state.
[25]
Medical[edit]
The primary
therapeutic use of nicotine is in treating nicotine dependence in order to eliminate
smoking with the damage it does to health. Controlled levels of nicotine are given to patients through
gums,
dermal patches, lozenges, inhalers, electronic/substitute cigarettes or nasal sprays in an effort to wean them off their dependence. A 2018
Cochrane Collaboration review found high quality evidence that all current forms of nicotine replacement therapy (gum, patch, lozenges, inhaler, and nasal spray) therapies increase the chances of successfully quitting smoking by
50–60%, regardless of setting,.
[33]
In contrast to recreational nicotine products, which have been designed to maximize the likelihood of addiction, nicotine replacement products (NRTs) are designed to minimize addictiveness.
[32]:112 The more quickly a dose of nicotine is delivered and absorbed, the higher the addiction risk.
[23]
Pesticide[edit]
Nicotine has been used as an
insecticide since at least the 1690s, in the form of tobacco extracts
[34] (although other components of tobacco also seem to have pesticide effects).
[35] Nicotine pesticides have not been commercially available in the US since 2014,
[36] and homemade pesticides are banned on organic crops
[37] and counterrecommended for small gardeners.
[38] Nicotine pesticides have been banned in the EU since 2009.
[39]Foods are imported from countries in which nicotine pesticides are allowed, such as China, but foods may not exceed maximum nicotine levels.
[39][40] Neonicotinoids, which are derived from and structurally similar to nicotine, are widely used as agricultural and veterinary pesticides as of 2016.
[41][34]
Enhancing performance[edit]
Contraindications[edit]
Side effects[edit]
Nicotine is not harmless,
[55] but it is safer than inhaled tobacco smoke.
[56] As medicine, nicotine is used to help with
quitting smoking and has good
safety in this form.
[26] The accepted medical position in 2007 was that nicotine itself poses few health risks, except among certain vulnerable groups
[27] such as youth,
[28] but the ideal course of action for smokers is to quit all nicotine use.
[57]
The common side effects from nicotine exposure are listed in the table below. Serious adverse events due to the use of nicotine replacement therapy are extremely rare.
[33]Common side effects of nicotine use according to route of administration and dosage form
| Associated side effects of nicotine | Sources |
|---|
| Buccal | Nicotine gum | Indigestion, nausea, hiccups, traumatic injury to oral mucosa or teeth, irritation or tingling of the mouth and throat, oral mucosal ulceration, jaw-muscle ache, burping, gum sticking to teeth, unpleasant taste, dizziness, lightheadedness, headache, and insomnia. | [33][54] |
| Buccal | Lozenge | Nausea, dyspepsia, flatulence, headache, upper respiratory tract infections, irritation (i.e., a burning sensation), hiccups, sore throat, coughing, dry lips, and oral mucosal ulceration. | [33][54] |
| Transdermal | Transdermal
patch | Application site reactions (i.e., pruritus, burning, or erythema), diarrhea, dyspepsia, abdominal pain, dry mouth, nausea, dizziness, nervousness or restlessness, headache, vivid dreams or other sleep disturbances, and irritability. | [33][54][58] |
| Intranasal | Nasal spray | Runny nose, nasopharyngeal and ocular irritation, watery eyes, sneezing, and coughing. | [33][54][59] |
| Oral inhalation | Inhaler | Dyspepsia, oropharyngeal irritation (e.g., coughing, irritation of the mouth and throat), rhinitis, and headache. | [33][54][60] |
| All (nonspecific) | Peripheral vasoconstriction, tachycardia (i.e., fast heart rate), elevated blood pressure, and increased alertness and cognitive performance. | [54][59] |
Cardiovascular system[edit]
Low levels of nicotine stimulate cell proliferation, while high levels are cytotoxic.[66] Nicotine increases cholinergic signaling and adrenergic signaling in colon cancer cells,[67] thereby impeding apoptosis (programmed cell death), promoting tumor growth, and activating growth factors and cellular mitogenic factors such as 5-lipoxygenase (5-LOX), and epidermal growth factor (EGF). Nicotine also promotes cancer growth by stimulating angiogenesis and neovascularization.[68][69] In cancer cells, nicotine promotes the epithelial–mesenchymal transition which makes the cancer cells more resistant to drugs that treat cancer.[70]Although nicotine does not cause cancer in humans,
[29] it is unclear whether it functions as a
tumor promoter as of 2012.
[64] A 2018 report by the
National Academies of Sciences, Engineering, and Medicineconcludes, “[w]hile it is biologically plausible that nicotine can act as a tumor promoter, the existing body of evidence indicates this is unlikely to translate into increased risk of human cancer.”
[65]
Fetal development and breastfeeding[edit]
Overdose[edit]
It is unlikely that a person would overdose on nicotine through smoking alone. The US
Food and Drug Administration (FDA) stated in 2013 that there are no significant safety concerns associated with the use of more than one form of
over-the-counter (OTC)
nicotine replacement therapy at the same time, or using OTC NRT at the same time as another nicotine-containing product, like cigarettes.
[73] The
median lethal dose of nicotine in humans is unknown.
[31][19] Nevertheless, nicotine has a relatively high
toxicity in comparison to many other alkaloids such as
caffeine, which has an LD
50 of 127 mg/kg when administered to mice.
[74] At sufficiently high doses, it is associated with nicotine poisoning,
[32] which, while common in children, rarely results in significant morbidity or death.
[30]
The initial symptoms of a nicotine overdose typically include
nausea, vomiting, diarrhea,
hypersalivation, abdominal pain,
tachycardia (rapid heart rate),
hypertension (high blood pressure),
tachypnea (rapid breathing), headache, dizziness,
pallor (pale skin), auditory or visual disturbances, and perspiration, followed shortly after by marked
bradycardia (slow heart rate),
bradypnea (slow breathing), and
hypotension (low blood pressure).
[30] Respiratory stimulation (i.e., tachypnea) is one of the primary
signs of nicotine poisoning.
[30] At sufficiently high doses,
somnolence (sleepiness or drowsiness),
confusion,
syncope (loss of consciousness from fainting),
shortness of breath, marked
weakness,
seizures, and
coma may occur.
[5][30] Lethal nicotine poisoning rapidly produces seizures, and death – which may occur within minutes – is believed to be due to
respiratory paralysis.
[30]
Reinforcement disorders[edit]
ΔFosB accumulation from excessive drug use
|
Normal between-cigarettes discontinuation, in unrestricted smokers, causes mild but measurable nicotine withdrawal symptoms.
[80] These include mildly worse mood, stress, anxiety, cognition, and sleep, all of which briefly return to normal with the next cigarette.
[80] Smokers have worse mood than they would have if they were not nicotine-dependent; they experience normal moods only immediately after smoking.
[25] Nicotine dependence is associated with poor sleep quality and shorter sleep duration among smokers.
[81][82]
In dependent smokers, withdrawal causes impairments in memory and attention, and smoking during withdrawal returns these cognitive abilities to pre-withdrawal levels.
[83] The temporarily increased cognitive levels of smokers after inhaling smoke are offset by periods of cognitive decline during nicotine withdrawal.
[80] Therefore, the overall daily cognitive levels of smokers and non-smokers are roughly similar.
[80]
Toxicity[edit]
Drug interactions[edit]
Pharmacodynamic[edit]
Pharmacokinetic[edit]
Pharmacology[edit]
Pharmacodynamics[edit]
Central nervous system[edit]

Effect of nicotine on dopaminergic neurons.
By binding to
nicotinic acetylcholine receptors in the brain, nicotine elicits its psychoactive effects and increases the levels of several
neurotransmitters in various brain structures – acting as a sort of "volume control."
[92][93] Nicotine has a higher affinity for nicotinic receptors in the brain than those in
skeletal muscle, though at toxic doses it can induce contractions and respiratory paralysis.
[94] Nicotine's selectivity is thought to be due to a particular amino acid difference on these receptor subtypes.
[95] Nicotine is unusual in comparison to most drugs, as its profile changes from
stimulant to
sedative with increasing
dosages, a phenomenon known as "Nesbitt's paradox" after the doctor who first described it in 1969.
[96][97] At very high doses it dampens
neuronal activity.
[98] Nicotine induces both behavioral stimulation and anxiety in animals.
[5] Research into nicotine's most predominant metabolite,
cotinine, suggests that some of nicotine's psychoactive effects are mediated by cotinine.
[99]
Sympathetic nervous system[edit]
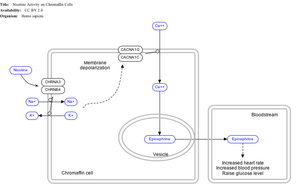
Effect of nicotine on chromaffin cells.
Nicotine also activates the
sympathetic nervous system,
[104] acting via
splanchnic nerves to the adrenal medulla, stimulating the release of epinephrine. Acetylcholine released by preganglionic sympathetic fibers of these nerves acts on nicotinic acetylcholine receptors, causing the release of epinephrine (and norepinephrine) into the
bloodstream.
Adrenal medulla[edit]
Pharmacokinetics[edit]

Urinary metabolites of nicotine, quantified as average percentage of total urinary nicotine.
[106]
The amount of nicotine absorbed by the body from smoking can depend on many factors, including the types of tobacco, whether the smoke is inhaled, and whether a filter is used. However, it has been found that the nicotine yield of individual products has only a small effect (4.4%) on the blood concentration of nicotine,
[109] suggesting "the assumed health advantage of switching to lower-tar and lower-nicotine cigarettes may be largely offset by the tendency of smokers to compensate by increasing inhalation".
Nicotine has a half-life of 1–2 hours.
Cotinine is an active metabolite of nicotine that remains in the blood with a half-life of 18–20 hours, making it easier to analyze.
[110]
Nicotine is
metabolized in the
liver by
cytochrome P450 enzymes (mostly
CYP2A6, and also by
CYP2B6) and
FMO3, which selectively metabolizes (
S)-nicotine. A major metabolite is
cotinine. Other primary metabolites include nicotine
N'-oxide, nornicotine, nicotine isomethonium ion, 2-hydroxynicotine and nicotine glucuronide.
[111] Under some conditions, other substances may be formed such as
myosmine.
[112]
Chemistry[edit]
NFPA 704
fire diamond |
|---|
The fire diamond hazard sign for nicotine. [114]
|
Nicotine is a
hygroscopic, colorless to yellow-brown, oily liquid, that is readily soluble in alcohol, ether or light petroleum. It is
miscible with
water in its
base form between 60 °C and 210 °C. As a
nitrogenous base, nicotine forms
salts with
acids that are usually solid and water-soluble. Its
flash point is 95 °C and its auto-ignition temperature is 244 °C.
[115] Nicotine is readily volatile (
vapor pressure 5.5 ㎩ at 25 ℃) and dibasic (K
b1=1×10⁻⁶, K
b2=1×10⁻¹¹).
[116] On exposure to ultraviolet light or various oxidizing agents, nicotine is converted to nicotine oxide, nicotinic acid (vitamin B3), and methylamine.
[117]
Nicotine is
optically active, having two
enantiomeric forms. The naturally occurring form of nicotine is
levorotatory with a
specific rotation of [α]
D=–166.4° ((−)-nicotine). The
dextrorotatory form, (+)-nicotine is physiologically less active than (−)-nicotine. (−)-nicotine is more toxic than (+)-nicotine.
[118] The salts of (+)-nicotine are usually dextrorotatory. The hydrochloride and sulphate salts become optically inactive if heated in a closed vessel above 180 °C.
[117]
Occurrence[edit]
The amounts of nicotine of tomato varieties lowered substantially as the fruits ripened.
[12] Nicotine content in tea leaves is greatly inconsistent and in some cases considerably greater than in the Solanaceae fruits.
[12] A 1999 report found "In some papers it is suggested that the contribution of dietary nicotine intake is significant when compared with exposure to ETS [environmental tobacco smoke] or by active smoking of small numbers of cigarettes. Others consider the dietary intake to be negligible unless inordinately large amounts of specific vegetables are consumed."
[12] The amount of nicotine eaten per day is roughly around 1.4 and 2.25
µg/day at the 95th percentile.
[12] These numbers may be low due to insufficient food intake data.
[12] Since the amounts of nicotine from the Solanum family including potato, tomato, eggplant, and from the Capsicum family vary in the parts per billion, they are tough to measure.
[121]
Biosynthesis[edit]
The biosynthetic pathway of nicotine involves a coupling reaction between the two cyclic structures that compose nicotine. Metabolic studies show that the
pyridine ring of nicotine is derived from
niacin (nicotinic acid) while the pyrrolidone is derived from
N-methyl-Δ
1-pyrrollidium cation.
[122][123] Biosynthesis of the two component structures proceeds via two independent syntheses, the NAD pathway for niacin and the tropane pathway for
N-methyl-Δ
1-pyrrollidium cation.
The NAD pathway in the genus
Nicotiana begins with the oxidation of aspartic acid into α-imino succinate by aspartate oxidase (AO). This is followed by a condensation with
glyceraldehyde-3-phosphate and a cyclization catalyzed by quinolinate synthase (QS) to give
quinolinic acid. Quinolinic acid then reacts with phosphoriboxyl pyrophosphate catalyzed by quinolinic acid phosphoribosyl transferase (QPT) to form niacin mononucleotide (NaMN). The reaction now proceeds via the NAD salvage cycle to produce niacin via the conversion of
nicotinamide by the enzyme
nicotinamidase.
[citation needed]
The
N-methyl-Δ
1-pyrrollidium cation used in the synthesis of nicotine is an intermediate in the synthesis of tropane-derived alkaloids. Biosynthesis begins with
decarboxylation of
ornithine by ornithine decarboxylase (ODC) to produce
putrescine. Putrescine is then converted into
N-methyl putrescine via
methylation by SAM catalyzed by putrescine
N-methyltransferase (PMT).
N-methylputrescine then undergoes
deamination into 4-methylaminobutanal by the
N-methylputrescine oxidase (MPO) enzyme, 4-methylaminobutanal then spontaneously cyclize into
N-methyl-Δ
1-pyrrollidium cation.
[citation needed]
The final step in the synthesis of nicotine is the coupling between
N-methyl-Δ
1-pyrrollidium cation and niacin. Although studies conclude some form of coupling between the two component structures, the definite process and mechanism remains undetermined. The current agreed theory involves the conversion of niacin into 2,5-dihydropyridine through 3,6-dihydronicotinic acid. The 2,5-dihydropyridine intermediate would then react with
N-methyl-Δ
1-pyrrollidium cation to form
enantiomerically pure (−)-nicotine.
[124]
Detection in body fluids[edit]
Nicotine can be quantified in blood, plasma, or urine to confirm a diagnosis of poisoning or to facilitate a medicolegal death investigation. Urinary or salivary cotinine concentrations are frequently measured for the purposes of pre-employment and health insurance medical screening programs. Careful interpretation of results is important, since passive exposure to cigarette smoke can result in significant accumulation of nicotine, followed by the appearance of its metabolites in various body fluids.
[125][126] Nicotine use is not regulated in competitive sports programs.
[127]
History, society, and culture[edit]
Nicotine is named after the tobacco plant
Nicotiana tabacum, which in turn is named after the
French ambassador in
Portugal,
Jean Nicot de Villemain, who sent tobacco and seeds to
Paris in 1560, presented to the French King,
[135] and who promoted their medicinal use. Smoking was believed to protect against illness, particularly the plague.
[135]
Tobacco was introduced to
Europe in 1559, and by the late 17th century, it was used not only for
smoking but also as an
insecticide. After
World War II, over 2,500 tons of nicotine insecticide were used worldwide, but by the 1980s the use of nicotine insecticide had declined below 200 tons. This was due to the availability of other insecticides that are cheaper and less harmful to
mammals.
[14]
The nicotine content of popular American-brand cigarettes has increased over time, and one study found that there was an average increase of 1.78% per year between the years of 1998 and 2005.
[136]
Legal status[edit]
In the European Union, the minimum age to purchase nicotine products is 18. However, there is no minimum age requirement to use tobacco or nicotine products.[137]In the United States, nicotine products and Nicotine Replacement Therapy products like Nicotrol are only available to persons 18 and above; proof of age is required; not for sale in vending machine or from any source where proof of age cannot be verified. In some states
[where?], these products are only available to persons over the age of 21.
[medical citation needed][where?]
In media[edit]
In some
anti-smoking literature, the harm that tobacco smoking and nicotine addiction does is personified as Nick O'Teen, represented as a humanoid with some aspect of a cigarette or cigarette butt about him or his clothes and hat.
[138] Nick O'Teen was a villain that was created for the
Health Education Council.
[138]
Research[edit]
Central nervous system[edit]
While acute/initial nicotine intake causes activation of neuronal nicotine receptors, chronic low doses of nicotine use leads to desensitisation of those receptors (due to the development of tolerance) and results in an antidepressant effect, with early research showing low dose nicotine patches could be an effective treatment of
major depressive disorder in non-smokers.
[139]
Though tobacco smoking is associated with an increased risk of
Alzheimer's disease,
[140] there is evidence that nicotine itself has the potential to prevent and treat Alzheimer's disease.
[141]
Immune system[edit]
Optopharmacology[edit]
A
photoactivatable form of nicotine, which releases nicotine when exposed to
ultraviolet light with certain conditions, has been developed for studying nicotinic acetylcholine receptors in brain tissue.
[143]
 #PROBEGGER
#PROBEGGER